Summary: QMS in pharma is a collection of practices, methodologies, and processes that ensure excellent product quality. Its primary objective is to prevent people and processes from diverting from quality standards like ICH Q10 and ISO. An example of QMS is process validation studies of pharma drugs that consist of data that ensures the drug manufacturing process is carried out according to pre-defined quality steps.
What is QMS in Pharma?
QMS in pharma industry ensures product lifecycle management stages like manufacturing and product testing ace quality standards.
It includes the following drug manufacturing stages:
- Method development
- Utility system
- Facilities
- Formulation
- Equipment
QMS in the pharmaceutical industry ensures the final product adheres to customer requirements and rules set by regulatory bodies. QMS in Pharma uses superior monitoring methods like Quality Assurance to provide the best product quality and documents each problem with its solution for future reference.
List of the applicable pharmaceutical QMS industry standards
QMS derives its definition and applicability based on several other approved standards. The ability of a pharmaceutical company to adhere to these standards is based on numerous factors, such as product type, geographical and demographical data, and target segment.
Here’s a list of the most common standards applicable to the Pharmaceutical Quality Management System (QMS in Pharma):
1. Current Good Manufacturing Practice (cGMP)
The Food and Drug Administration (FDA), a U.S. federal agency, enforces cGMP that monitors the safe production of drugs. It prevents the mix-up of one product with another and also checks for any contamination. This process ensures that manufactured products are fit for human consumption.
The FDA is the governing body that inspects whether an accredited supplier is strictly adhering to the cGMP guidelines. If it identifies a drug manufacturer wilfully flouting rules or involved in drug adulteration, it orders the manufacturer to recall the contaminated product. Moreover, if the manufacturer fails to comply with FDA directions, it results in heavy penalties or jail.
2. International Organization for Standardization (ISO)
The ISO develops frameworks and quality standards for multiple industries, including pharmaceuticals. Currently, the standard for Quality Management System is ISO 9001:2015, which is an improved version of ISO 9001:2005.
ISO is an international body that creates standards and frameworks but doesn’t perform certification. Instead, independent contractors carry out the inspection and certification process, ensuring a drug manufacturer complies with ISO standards.
All the standards for certification bodies in the ISO certification process are developed by ISO’s Committee on Conformity Assessment (CASCO).
3. 21 CFR Part 211
21 CFR Part 211 consists cGMP guidelines for finished pharmaceuticals. The following list shows the topics this regulation covers:
- Quality buildings and facilities
- Process equipment
- Warehouse requirements
- Quality control
- Ventilation and filtration system
- Qualification and skills of the personnel involved
- Product labels
4. 21 CFR Part 11 for electronic signatures and records
IoT devices allow recording process variables automatically without any human intervention. It increases efficiency and eliminates human-induced errors and tampering with data.
The 21 CFR Part 11 contains regulations that describe how electronic records are generated, stored, and maintained. It also determines how authorized stakeholders and supervisors would authorize these documents.
5. ICH guideline Q10
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) creates regulations for quality management systems. It consists of professionals from the manufacturing and regulatory bodies.
It frames guidelines on topics such as:
- Impurities
- Validation
- Risk Management
The Q10 guideline doesn’t provide a different framework for pharmaceutical quality standards. Instead, it frames guidelines based on established standards, such as:
- ICH Q8 Pharmaceutical Development
- ISO
- ICH Q9 Quality Risk Management
- cGMP
Fundamental principles of ISO management
ISO lays out seven quality management principles. An organization can choose to adopt all or some of them.
Let’s learn about them in detail.
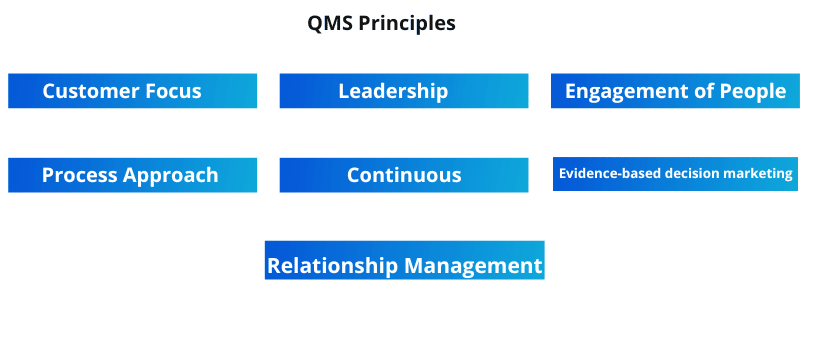
QMP 1 — Customer Focus
The customer is the primary area of concern in the first principle of QMS in pharma. It says that the quality management processes should be optimized enough to meet customer requirements. It has two essential benefits. Firstly, the loyal customers remain intact, which increases the company’s revenues. And secondly, it builds a positive image in the market, making it easy to acquire new customers.
QMP 2 — Leadership
The second principle of QMS for the pharmaceutical industry helps frame guidelines, enabling business stakeholders to collaborate to achieve collective organizational goals. QMP 2 also helps set quality goals for attaining high product quality standards.
Moreover, it facilitates smooth communication between various business stakeholders and departments and provides training opportunities, ensuring employees are updated with the latest regulatory requirements.
Leadership is essential in establishing a top-notch quality management system (QMS). The following points explain how:
- Quality Policy and Quality Planning: An organization’s leadership is entrusted with the duty to create quality policy and ensure that it’s aligned with the company’s vision and mission.
- Communication: The company’s leadership needs to ensure that different departments generate and easily share critical business data.
- Commitment: Leadership is responsible for establishing a working framework for a pharmaceutical quality management system. Moreover, they should also enlist the duties and functions of each individual while implementing a QMS in pharma.
- Review: The leadership should critically examine the product quality and process performance and identify quality defects.
- Resource management: The leadership ensures business departments have access to adequate resources to ensure high-level product quality. Moreover, they also minimize resource wastage.
QMP 3 — Engagement of People
Employee engagement ensures greater employee loyalty, lower absenteeism, higher retention, and better employee health. It can be done across different levels of the hierarchy. And the best way to evaluate people’s engagement level and their commitment to product quality is by conducting surveys. QMS in pharma helps conducts surveys quickly and efficiently.
QMP 4 — Process Approach
The primary requirement of the Quality Management Principle (QMP) is that organizations should define interconnected steps impacting each other’s performance. Risks in the system are identified on two levels — individual and the system as a whole.

QMP 5 — Improvement
Improvement of Quality Management System (QMS) in the pharmaceutical industry focuses primarily on two sectors, product development and regulations.
The senior management must review and track the performance of the pharmaceutical quality management software using the following systems:
- Regulatory body reports
- Feedback system
- CAPA
- Supplier audits
- Risk assessments
QMP 6 — Evidence-Based Decision Making
This principle of QMS in pharma talks about taking a practical approach while making decisions for the pharmaceutical industry. However, decision-makers are often confused due to the vulnerabilities, complexities, and risks that real-world processes pose.
And as the number of variables increases, leaders need to examine each data point and ensure they are accurate. Thus, leaders should possess advanced data processing and evaluation techniques, allowing them to reveal hidden insights from multiple data sources.
QMP 7 — Relationship Management
An organization’s success depends on how efficiently it manages its relationship with multiple stakeholders, including employees, suppliers, distributors, and vendors. It helps create common goals that each party can work towards in unison.
Each stakeholder impacts the relationship uniquely, and therefore it’s crucial to prioritize the relationship level. It would be best to begin by paying close attention to short and long-term goals. Once you brainstorm a flawless strategy, it’s time to share critical resources, expertise, and data. To strengthen trust, you can also provide feedback and share tips and tricks on how they can improve their performance.
3 Important features of the pharmaceutical management systems
A pharmaceutical management system has three primary elements. These elements can be incorporated based on product lifecycle requirements without adhering to any particular order.
The three elements are:
- Change management system
- Product quality monitoring system
- Corrective Action and Prevention Action (CAPA) System
1. Change management system
ICH and ISO boost innovation in existing processes by enhancing product quality. A change control management system allows pharmaceutical companies to examine, check, adjust, and authorize the change.
If the change is incorporated in the process after receiving the regulatory clearances, it’s submitted to the regulatory body for approval.
Consider the following example. The supply chain team suggests changes in the packaging size to decrease the logistical expenses. Then, the quality control department checks the proposed modifications based on terms and conditions set by the regulatory bodies and approves the change. Ultimately, the R&D department creates new prototypes by applying these changes. Once the new packaging size is available, the supply chain team purchases a small quantity of such materials to check for its success. If the logistics costs reduce, the supply chain team shares the data with other departments.
The production department makes relevant changes in the pharmaceutical standard operating procedure (SOP) after receiving the changes from the supply chain team. Further, these changes are shared with the Production, Quality, and Engineering department that officially updates the change control format. Finally, the senior management critically analyzes these changes and, if found valuable, makes them a part of the company’s policy.
QMS in pharma simplifies the process by automatically tracking changes to product process, SOP, and instructions. It also facilitates the changes through the approval process, ensuring they are applied quickly and efficiently.
<<<Also Read: Change Management: Keep your employees excited about a new ERP system>>>
2. Product Quality Monitoring System
The product quality monitoring systems allow an organization to control and use resources sustainably.
Risk examination methods help identify defects in the process components. Pharma companies can also use customer feedback and specialized tools to analyze process parameters.
QMS in pharma efficiently manages routing, data collection, and resolves issues. This way, pharma companies can perform safety monitoring of business processes and products quickly and efficiently.
3. Corrective Action and Prevention Action (CAPA) System
CAPA helps identify the cause of the problems and prevents them from happening again. It can be applied to problems that arise outside and inside the organization.
The CAPA structure varies from one organization to another, but the following points remain almost the same:
- Steps to prevent the same problem from recurring.
- Identifying the department where the problem exists.
- Determining the exact details of the fault.
- Finding the impact of the fault on the process or product.
- Identifying the solution to the identified problem.
- The date when the corrective & preventive action will be executed.
- The review date to analyze the effectiveness of the correction action.
QMS in pharma software contains a CAPA module that automates follow-ups, approvals, notifications, and data collection, helping manage corrective and preventive actions quickly and efficiently.





